SiMD - Software IN a medical device (SiMD) means that the software is part of another medical device and helps it function in some way.
Medical device software development refers to the process of planning, designing, developing, and testing software applications intended for use in or as medical devices
Whether you are a MedTech startup seeking your first FDA approval, a mid-sized company going digital, or a leading medical device manufacturer looking to outsource your engineering needs, we have you covered. Our medical device software development services are ISO 13485 and IEC 62304 compliant and backed by over 15 years of experience in the healthcare industry. We rely on proven software architecture and project management practices to design and build secure, MDR- and FDA-cleared SaMDs and SiMDs.
SaMD - Software AS a medical device (SaMD) means that the software itself is the device.
Our tailored approach
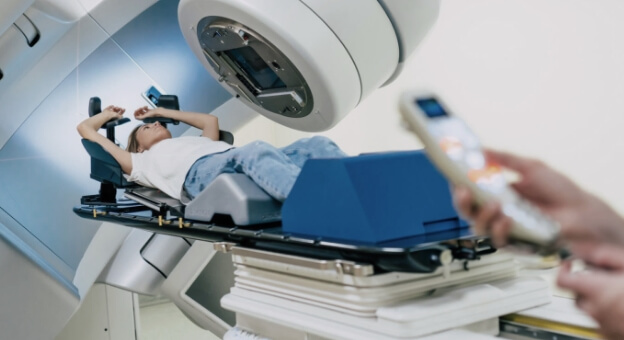
We enjoy working on projects in the medical field with a quality-driven approach to medical device software development. Our track record includes diagnostic devices, health monitors, and C-classified medical devices such as a 6D patient positioning system under a linear accelerator, providing quality- focused software development and testing.
Our team can work alongside existing in-house teams or be your provider of the full engineering service stack for medical NPD. If needed, we can conduct a skills gap analysis and recommend a suitable team to fill those gaps.
We also have a verification and validation team that can integrate with your larger multidisciplinary team. If required, we can work with clients to explore the creation of a custom automated test solution.
Our compliant process
Our ISO 9001 certified QMS is aligned with ISO 13485, but does not have said certification. We made a conscious decision to do this in order to take full advantage of the Agile software development methodology. For our medical device customers, we ensure that our processes meet the requirements of ISO 13485, which has been confirmed several times by second party audits conducted by our clients. What you get is a development team following your QMS instead of their own.
Whether your product requires EU or FDA approval, we have a harmonized and compliant approach to both.
Case examples
Our ideal customer profile
-
Medical device manufacturers
-
Healthcare software product companies and startups
-
Pharmaceutical, biotech, and life science companies
-
Medical device manufacturers 2
Our medical software development service vs.
Contractors
Rubedos is always more cost effective. You’ll only need to onboard us as part of your procurement process, rather than recruiting and payroll. We’ll always have people available to work on your project, helping you stay on schedule. Meanwhile, a contractor, despite an average day rate of €640, still needs a team leader to keep them on track, not to mention sick leave and holidays.
In-house team
Rubedos is more cost effective in the first year and less cost effective as you build your in-house team. If you hire the right people and take care to learn how to establish good processes, it will take an in-house team 1 to 2 years to become as productive as our high-performing team. Once that happens, they’ll be a better value.
Cost saving steps
New product development process
Feasibility study
Perform a detailed feasibility study prior to starting any cost intensive phase
Rapid prototyping
Develop a rapid prototype, provided the feasability study report shows promise
Prototype validation
Use standard components whenever possible to cut corners on expensive R&D
Grade design
Make sure the prototype gets validated prior to applying design management process